Sight for sore eyes: New lenses slow myopia in kids
A Breakthrough in Myopia Treatment
In late September, the U.S. Food and Drug Administration (FDA) made a groundbreaking announcement that could significantly impact the lives of millions of children. The FDA authorized the marketing of a new eyeglass lens designed to correct myopia, commonly known as nearsightedness. This innovative lens not only addresses myopia with or without astigmatism but also slows the progression of the condition in children aged 6 to 16 at the time of their initial diagnosis.
Dr. Michelle Tarver, Director of the Center for Devices and Radiological Health, emphasized the importance of this development. “As a practicing ophthalmologist, I see firsthand the lifelong impact that vision problems can have on an individual,” she said in a statement. “This authorization brings to market a treatment option that may meaningfully reduce the likelihood of severe eyesight issues later in adult life, while also being easier to use and lower risk than the currently authorized devices that slow the progression of myopia in children.”
The lens, developed by Essilor, a company based in Paris, France, is called the Essilor Stellest eyeglass lens. Prior to the Stellest, there was only one FDA-approved device for slowing myopia progression—contact lenses for children aged 8 to 12. The Stellest represents a significant advancement, offering a safer and more accessible alternative for younger children and those who cannot wear contact lenses.
Limited Treatments for Myopia in Children
Francesco Milleri, Chairman and CEO of parent company EssilorLuxottica, highlighted the significance of this innovation. “This lens technology evolves the traditional corrective lenses into a true medical treatment, and it marks the beginning of a new era for eyecare professionals in addressing myopia,” he stated. “We are thrilled to have been granted FDA’s market authorization for Essilor Stellest in the U.S., bringing it to young patients in need. This groundbreaking innovation is a major milestone on our mission to empower people and allow them to greater control their health.”
According to both Essilor and the FDA, the Stellest lenses, when worn for 12 hours daily, slow the progression of myopia by an average of 67% compared to other single-vision lenses. After two years of usage, there was a 71% slowdown in myopia progression. The FDA noted that “the Essilor Stellest eyeglass lenses can fill the gap for children 6 to 7 years old or for children who are unable to wear contacts. Essilor Stellest eyeglass lenses are a lower risk device compared to contact lenses and do not have adverse events (such as infections) that may be associated with the use of contact lenses.”
How the Lenses Work
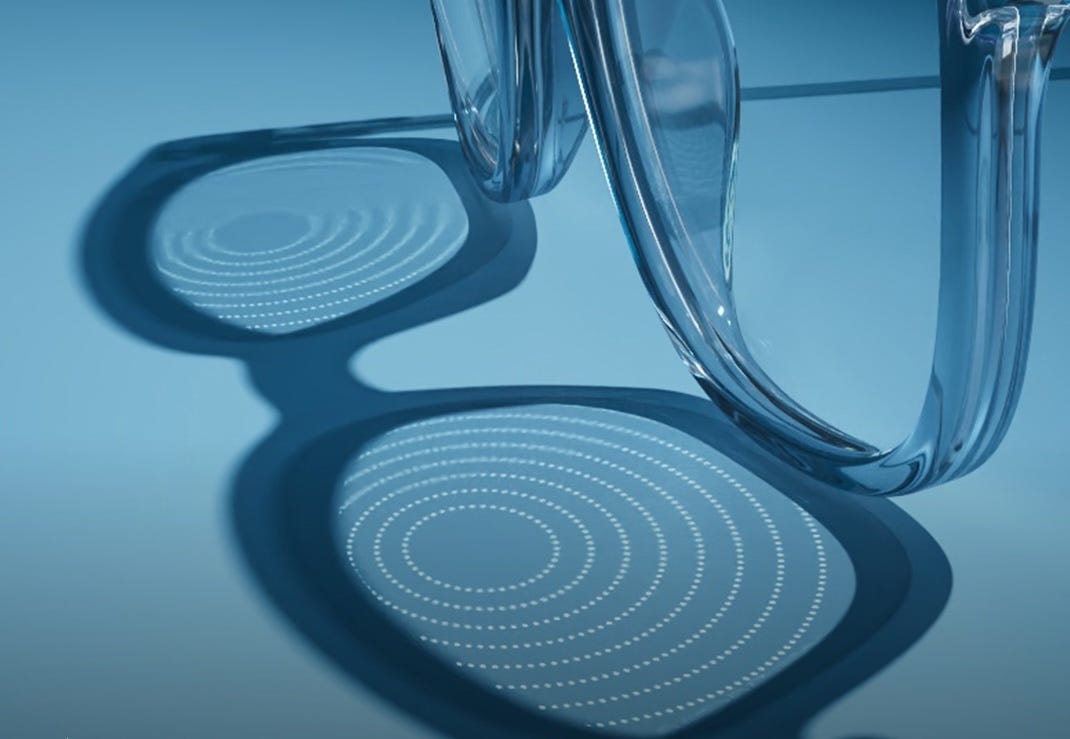
The FDA explained that the Essilor Stellest eyeglass lenses have a clear 9mm diameter area in the center, surrounded by rings of tiny, raised dots (peripheral lenslets) on the rest of the lens. These tiny, raised dots provide peripheral light defocus, which may help to slow the progression of myopia in children.
According to the National Institutes of Health, myopia occurs when the eye grows too long, and the image projected on the retina is poorly focused when a person is trying to view an image at a distance. CNN reported that “when a child first becomes nearsighted is when their vision is changing the fastest and their eye is growing most rapidly.”
Jeff Walline, a professor of optometry at Ohio State University, told CNN that “we know from longitudinal studies that a child’s myopia is progressing the fastest when you first see that they become nearsighted” and that’s why he urges parents to treat their child’s condition at the first indication that their vision has become compromised.
Essilor explained that its Stellest lens incorporates a technology it calls HALT (Highly Aspherical Lenslet Target). The company says that the HALT technology is comprised of “a constellation of 1,021 invisible lenslets. This constellation creates a signal in front of the retina that acts as a shield against eye elongation and, therefore, myopia progression.”
Clinical testing showed that after two years of usage, eye elongation—which causes myopia—was decreased by 53%. The FDA noted that “there were no serious adverse events reported in the clinical study. However, some subjects did report visual symptoms, such as blur and halos.”
Increasing Prevalence of Myopia
The National Eye Institute notes that several studies indicate that the prevalence of myopia is increasing in the U.S. and worldwide, with researchers projecting the trend will continue in the coming decades. The condition currently affects approximately 40% of the U.S. population, with the prevalence increasing rapidly among children and adolescents.
The National Eye Institute estimated that, by 2050, more than 50% of the world’s population may become myopic. When left untreated, or undertreated (meaning that the patient’s vision correction prescription isn’t updated), the condition can progress to “high myopia”—a disease that significantly increases the risk for sight-threatening complications, including retinal detachment, myopic maculopathy, glaucoma, and cataracts later in life.
- 100 Soal dan Kunci Jawaban Matematika SD Kelas 6 Semester 2 Kurikulum Merdeka 2026 - February 27, 2026
- Cara cek tagihan BPJS Kesehatan di HP, hindari menunggak - February 26, 2026
- Top Educated States in America: Is Pennsylvania Among Them? - February 26, 2026



Leave a Reply